We KNOW HOW to take care of animals!
Why are our products trusted by customers? Because the foundation for VET PLANET development is in the creation of our own unique products based on the latest scientific achievements.

Based on evidence
Only proven solutions
It is not coincidence that our brand, VET EXPERT, incorporates the term Based on evidence in its logo. This statement reflects the approach of creation of innovative products and solutions that due to our effort are available in veterinary clinics and at pet parents homes. There are products in our products that are not only effective but also innovative and first in their class. WE WERE THE FIRST IN THE WORLD to introduce innovations on a global scale:
-
+ TWIST OFFThe 1st in the world adaptation of TWIST OFF technology to veterinary pet products. The unique extremely palatable and convenient dosage formula of the TWIST OFF capsules is considered the friendliest form of supplementation by veterinarians and pet parents.
-
+ ProlactiNOThe 1st in the world product based on completely natural substances intended for female dogs with pseudo pregnancies.
-
+ RenalVetThe 1st in the world phosphorus chelator with active vitamin D, a preparation designed for dogs and cats to support proper kidney function.
-
+ SemeVetThe 1st in the world product for dogs that improves semen quality.
-
+ Stimuderm UltraThe 1st in the world line of products for the management of hair loss in dogs.
Biotech lab
Unique biotechnological laboratory
We have established a scientific centre where our Team – biotechnologists, molecular biologists and veterinary doctors – uses the most advanced technologies to develop innovative diagnostic tools.
We are proud to announce that our projects have been recognized and given support from the Polish National Centre of Research and Development.
We are open to collaborating with external entities – scientific research institutions and businesses. Interested? Contact us!

Own scientific journal
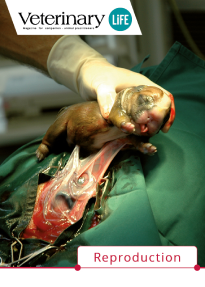
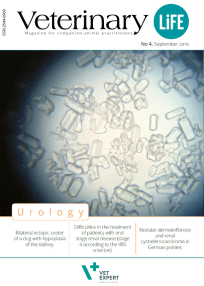
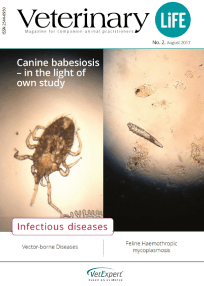
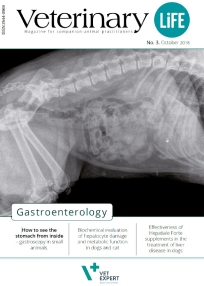
Among many exceptional things we are involved in, there is "Veterinary Life" - a specialized veterinary journal created for practicing veterinarians. We publish the magazine to share knowledge and facilitate the exchange of experiences.
Our invitation to publish in the pages of "Veterinary Life" is accepted by the best clinicians and academics.
“Veterinary Life” is also a platform to present the results of scientific research conducted using our products. If you want to develop, reach for “Veterinary Life”!

Exceptional educational platform
VET EXPERT ACADEMY provides top-notch veterinary training – we share with veterinarians and students the latest scientific achievements and innovative solutions in modern veterinary medicine.
The most outstanding experts, key worldwide opinion leaders - recognized authorities inform about the latest most advanced methods of dealing with diseases in dogs and cats.
VET EXPERT ACADEMY guarantees education at a global level.
Visit the platformOur development projects
We are the world's first to conduct a series of studies aimed at aiding in the fight against animal diseases.
POIR.04.01.04-00-0025/20
National Centre for Research and Development Operational Programme Smart Growth 2012-2020
Applicant: Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences
Project classification
Biotechnological and chemical processes, bioproducts, specialized chemistry products and environmental engineering.
The project is co-financed by the European Union from the European Regional Development Fund under the Smart Growth Operational Programme. The project is implemented as part of a competition by the National Centre for Research and Development: Industrial research and development work carried out by enterprises.
POIR.01.01.01-00-1715/20
National Centre for Research and Development Smart Growth Operational Programme 2014-2020
Applicant: BioScentia Sp. z o.o.
Project classification
Healthy society
The project is co-financed by the European Union from the European Regional Development Fund under the Smart Growth Operational Programme. The project is implemented as part of a competition by the National Centre for Research and Development. Industrial research and development work carried out by enterprises.
POIR.01.01.01-00-0267/18
National Centre for Research and Development Smart Growth Operational Programme 2014-2020
Applicant: VET PLANET
Project classification:
Innovative technologies, processes, and products in the agri-food and forestry sectors.
The project is co-financed by the European Union from the European Regional Development Fund under the Smart Growth Operational Programme. The project is implemented as part of a competition by the National Centre for Research and Development: Industrial research and development work carried out by enterprises.
POIR.01.01.01-00-1576/20
National Centre for Research and Development Smart Growth Operational Programme 2014-2020
Applicant: VET PLANET
Project classification:
Innovative technologies, processes, and products in the agri-food and forestry sectors.
The project is co-financed by the European Union from the European Regional Development Fund under the Smart Growth Operational Programme. The project is implemented as part of a competition by the National Centre for Research and Development: Industrial research and development work carried out by enterprises.
 European Union
European Union